LiF-armored lithium anode unlocks ultra-stable and fire-safe batteries
GA, UNITED STATES, December 11, 2025 /EINPresswire.com/ -- Lithium metal batteries hold the promise of exceptional energy density, yet their performance often collapses when high levels of flame-retardant additives are introduced for safety. The new study proposes a finely tuned strategy that stabilizes the lithium metal anode by building a LiF-rich artificial solid electrolyte interphase (SEI). This protective layer counters the corrosive effects caused by triphenyl phosphate (TPP) in flame-retardant gel polymer electrolytes. By pairing a dual-confinement electrolyte design with a pre-engineered LiF shield, the system maintains stable cycling across both mild and demanding conditions. The results point to a practical route for creating long-lasting, inherently fire-safe lithium metal batteries.
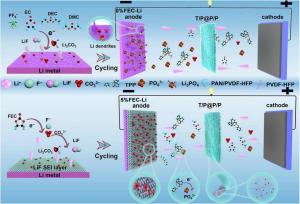
Lithium metal stands out as an anode material due to its exceptionally high theoretical capacity, yet its real-world use is challenged by dendrite growth, unstable interfacial chemistry, and the flammability of conventional electrolytes. Gel polymer electrolytes address some safety concerns but still rely on large quantities of flame retardants. Organic phosphates such as triphenyl phosphate (TPP) enhance fire resistance but tend to penetrate the SEI, triggering decomposition reactions that severely corrode lithium. At high concentrations, these additives increase safety at the cost of dramatically shortened battery life. Based on these challenges, there is a pressing need to develop interface and electrolyte designs that ensure both flame retardancy and long-term anode stability.
Researchers from Hebei University of Science and Technology, City University of Hong Kong, and Hainan University have reported a significant advancement in electrolyte–anode interface engineering in a study published on September 23, 2025, in Carbon Energy. The team designed a high-TPP-loading flame-retardant gel polymer electrolyte and paired it with a pre-formed LiF-rich solid electrolyte interphase (SEI) on lithium metal. This combined strategy suppresses corrosion, accelerates Li⁺ transport, and enables stable cycling even when the batteries are pushed to high current densities.
The team began by developing a gel polymer electrolyte containing 70 wt.% TPP using a coaxial electrospinning technique. This structure features a TPP / Poly(vinylidene fluoride–co–hexafluoropropylene) composite (TPP/PVDF-HFP) core encased within a PAN/PVDF-HFP shell, forming a dual-confinement design. Strong chemical interactions between PVDF-HFP and TPP limit molecular leakage, while the surrounding polymer shell provides physical containment. Together, these mechanisms maintain high flame retardancy while curbing the corrosive side reactions typically triggered by TPP.
To further fortify the anode interface, the researchers immersed lithium metal in a 5% FEC-containing electrolyte, producing a uniform and dense LiF-rich SEI layer. Multi-modal analyses—including UV–vis spectroscopy, TOF-SIMS, XPS, and AFM—showed that the engineered SEI blocks the penetration of TPP-derived species and substantially reduces the depth of anode corrosion. Beyond protection, the LiF layer enhances lithium-ion mobility, lowers the activation energy for interfacial transport, and promotes smooth, dendrite-free plating.
Electrochemical tests validated the design: Li||Li cells operated stably for 2400 hours at 0.5 mA cm⁻² and 1500 hours at 5 mA cm⁻². In full-cell configurations, LFP||Li cells retained 98.9% of their capacity after 1500 cycles at 1 C and preserved 81.7% capacity after 6000 cycles at 10 C—demonstrating exceptional endurance under fast-charging conditions.
“The study compellingly shows that precise interface engineering is essential to advancing both the safety and durability of lithium metal batteries,” said the lead corresponding scientist. “By integrating a dual-confinement flame-retardant electrolyte with a LiF-rich artificial SEI, we resolved the long-standing conflict between fire protection and anode stability. This approach not only halts the severe corrosion caused by phosphate-based additives but also improves lithium-ion transport, enabling reliable operation under high rates and extended cycling conditions.”
This combined SEI–electrolyte strategy represents a promising direction for developing high-performance, intrinsically safer lithium metal batteries. Its ability to sustain thousands of cycles at high current densities positions it well for electric vehicles, grid-level storage, aerospace systems, and next-generation flexible pouch cells. More broadly, the underlying design principle—merging chemical confinement, structural encapsulation, and deliberate SEI engineering—can be applied to other reactive anodes and high-voltage cathodes. As global demand for high-energy batteries intensifies alongside strict safety requirements, this approach may accelerate the practical adoption of lithium metal technologies.
References
DOI
10.1002/cey2.70077
Original Source URL
https://doi.org/10.1002/cey2.70077
Funding information
The National Natural Science Foundation of China (52404316, 52474325); The S&T program of Hebei Province (225A4404D); The Natural Science Foundation of Hainan Province (524RC475); The Collaborative Innovation Center of Marine Science and Technology of Hainan University (XTCX2022HYC14); The Xingtai City Natural Science Foundation (2023ZZ027).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.